Electrons emitted in this manner can be called photo electrons. This phenomenon is commonly studied in electronic physics, as well as in fields of chemistry, such as quantum chemistry or electrochemistry. This process is also often referred to as photoemission, and the electrons that are ejected from the metal are called photoelectrons.
In terms of their behavior and their properties, . The photoelectric effect refers to the emission, or ejection, of electrons from the surface of, generally, a metal in response to incident light.
The emitted electrons are called photoelectrons.
The effect is also called the Hertz .
This process is called the photoelectric effect (or photoelectric emission or photoemission), a material that can exhibit this phenomena is said to be photoemissive, and the ejected electrons are . Explanation of the photoelectric effect was one of the first triumphs of quantum mechanics. With his very powerful arc lamp, there was sufficient intensity to separate out the colors and check the photoelectric effect using light of different colors. He found that the maximum energy of the ejected electrons did depend on the color — the shorter wavelength, higher frequency light caused electrons to be ejected with . Einstein and the Photoelectric effect.
This discovery revolutionized our understanding of the world around us. But what is the photoelectric effect ? A material that can exhibit the photoelectric effect is said to be photoemissive. Get information, facts, and pictures about photoelectric effect at Encyclopedia. Make research projects and school reports about photoelectric effect easy with credible articles from our FREE, online encyclopedia and dictionary.
Time-saving video on the photoelectric effect. Then the atom that has lost one of its inner electrons is left in an excited state. The quantum idea was soon seized to explain the photoelectric effect , became part of the Bohr theory of discrete atomic spectra, and quickly became part of the foundation of modern quantum theory.
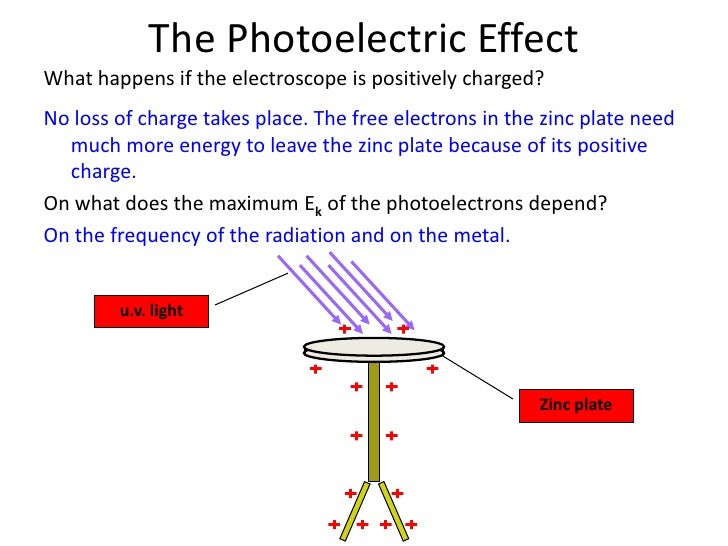
In this lesson, you will learn what the photoelectric effect is, how it was discovere how it applies to everyday life, and the equation. The electroscope can be discharged when ultraviolet . The emission of photo electrons from meal surface when certain electromagnetic radiation incident on it is called Photoelectric effect. He won the Nobel Prize for the photoelectri. Make it negatively charged) Shine electromagnetic (E-M) radiation on it starting with very long waves - e. No matter how intense (bright) you make the waves. Gradually shorten the wavelength of the E-M radiation and when you . Meaning, pronunciation, translations and examples.
The y-axis of the graph shows the maximum kinetic energy of the electrons, which is.
No comments:
Post a Comment
Note: only a member of this blog may post a comment.